LATEST LOAD - SEPTEMBER 2022
The following ADDN snapshot was provided as part of the most recent Benchmarking reports to 35 participating ADDN centres and represents current data in the ADDN Registry as of September 2022. It was produced using clinical and demographic data from ADDN sites as part of the most recent load between August and September 2022. For this particular load, data are only available for visits between 1/7/2021 and 31/12/2021 for 3 centres.
For each person with diabetes included below, visits for the 12-month period preceding the most recent visit are included in the reporting period. ADDN has no control over the representativeness or accuracy of patient data provided.
If you have any questions about benchmarking reports or data uploads, please contact our program team at ADDNinfo@unsw.edu.au.
inclusions
People with diabetes with at least one visit between 01/07/2021 and 30/06/2022 inclusive.
People with any type of diabetes: Type 1 diabetes (T1D); Type 2 diabetes (T2D); or Other.
exclusions
HbA1c measured within the first 3 months post-diagnosis.
Definitions
Age: Mean age for each individual patient calculated from all eligible visits within the reporting period.
Age Categories: Determined from mean age above.
Glycaemic Control: Mean HbA1c for each individual patient calculated from all eligible visits within the reporting period
Glycaemic Control Categories: Determined from mean HbA1c as above
Median HbA1c: Median using the mean HbA1c for each individual patient calculated from all eligible visits within the reporting period
DIY Regimen: Patients using a Do It Yourself pump/CGM combination (Looping)
HCL Regimen: Patients on a Hybrid Closed Loop pump regimen
Insulin Treatment: most recently recorded regimen within the reporting period; insulin pump (CSII); multiple daily injections (MDI, > 3 injections per day); twice daily injections (BD); other not specified; unknown (missing)
HbA1c conversion: For tables and graphs showing HbA1c as % only, the following can be used to roughly convert to mmol/mol
| % | mmol/mol |
|---|---|
| 7 | 53 |
| 8 | 64 |
| 9 | 75 |
| 10 | 85 |
| 12 | 107 |
| 14 | 130 |
addn centres INCLUDED IN THIS SNAPSHOT
Adult Centres (12)
Blacktown-Mt Druitt Hospital, Sydney, NSW
Campbelltown Hospital, Sydney, NSW
Liverpool Hospital, Sydney, NSW
Westmead Hospital, Sydney, NSW
Mater Hospital, Brisbane, QLD
Lyell McEwin and Modbury Hospitals, Adelaide, SA
St Vincent’s Hospital, Melbourne, VIC
Western Health Sunshine Hospital, Melbourne, VIC
Royal Melbourne Hospital, Melbourne, VIC
Fiona Stanley Hospital, Perth, WA
Waikato Hospital (Adults), Waikato, NZ
Waitemata North Shore Hospital, Waitemata, NZ *
* new centres for Sept 2022 (first data load)
Paediatric CentreS (23)
Canberra Hospital (Paediatrics), Canberra, ACT
John Hunter Children's Hospital, Newcastle, NSW
The Children’s Hospital at Westmead, Sydney, NSW
St George Hospital (Paediatrics), Sydney, NSW
Illawarra Shoalhaven Nowra, Nowra, NSW
Illawarra Shoalhaven Wollongong, Wollongong, NSW
Queensland Children’s Hospital, Brisbane, QLD
Women’s and Children’s Hospital, Adelaide, SA
Monash Children's Hospital, Melbourne, VIC
Royal Children’s Hospital, Melbourne, VIC
Barwon Health University Hospital, Geelong, VIC
Ballarat Health Services, Ballarat, VIC
Perth Children's Hospital, Perth, WA
Starship Children’s Hospital, Auckland, NZ
Wairau Hospital, Blenheim, NZ
Christchurch Hospital, Christchurch, NZ
Southern District Health Board, Dunedin, NZ
Hawkes' Bay Hospital, Hastings, NZ
Nelson Hospital, Nelson, NZ
Palmerston North Hospital, Palmerston North, NZ *
Timaru Hospital, Timaru, NZ
Waikato Hospital (Paediatrics), Waikato, NZ
Whangarei Hospital, Whangarei, NZ
ADDN Snapshot, T1D
This section is a general snapshot across ADDN and includes aggregated T1D data for both Paediatric and Adult centres.
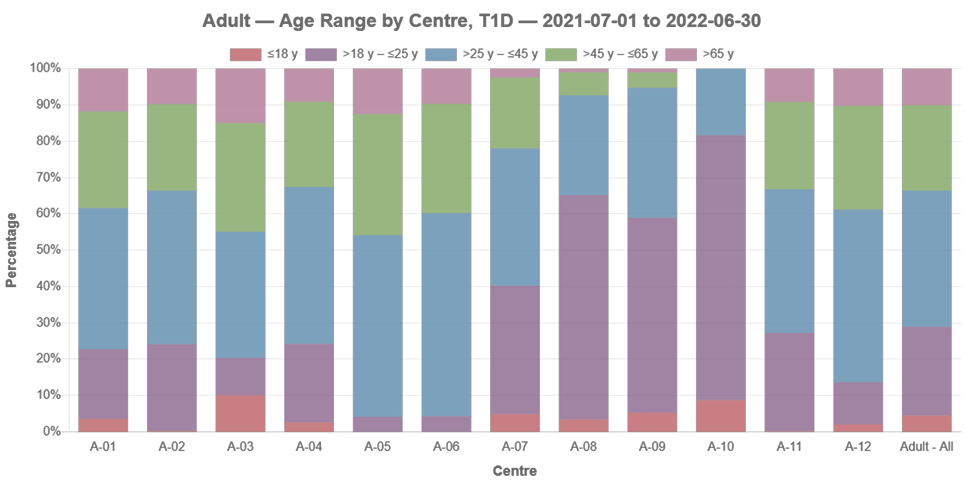
age range
region
glycaemic control
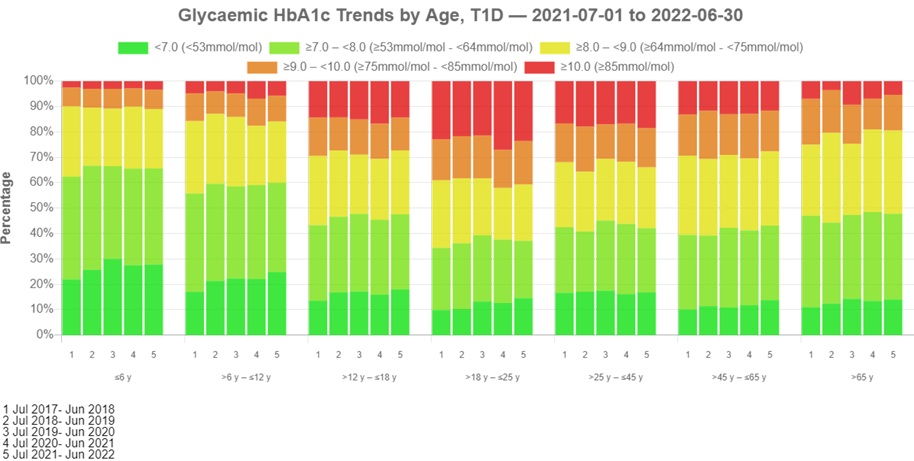
glycaemic hba1c trends by age
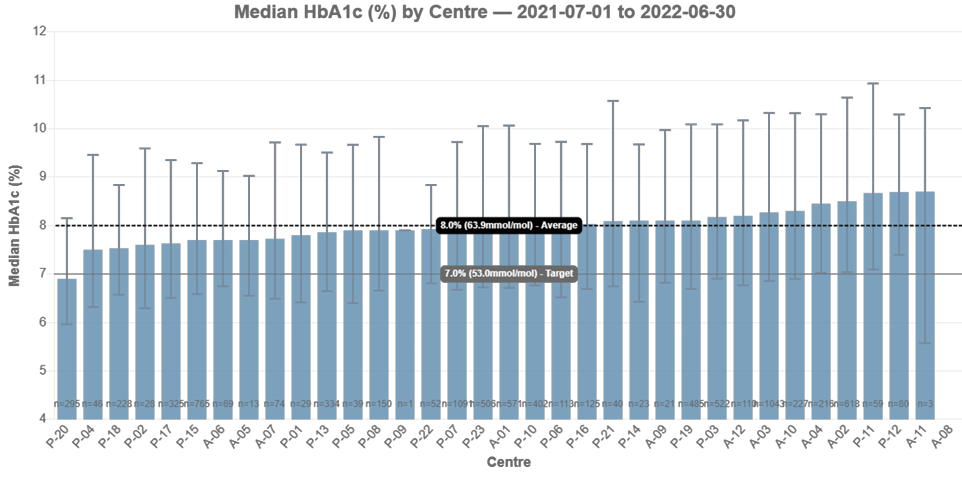
ADDN Centre Comparisons, T1D
This section continues the general snapshot across ADDN and includes T1D data for both Paediatric and Adult centres. Each centre has been reported separately and is de-identified.